Abstract
Introduction
The myeloproliferative neoplasms (MPNs) are clonal stem cell disorders characterized by hematopoietic stem/progenitor cell (HSPC) expansion and overproduction of mature blood cells. The acquired mutation JAK2V617F plays a central role in these disorders. The mechanisms responsible for MPN HSPC expansion are not fully understood. One hallmark of MPNs is megakaryocytic (MK) hyperplasia. Very recent evidence has implicated MKs in regulating HSPC activity. Since thrombopoietin (TPO) acting through its receptor MPL are key regulators of both megakaryopoiesis and HSPC activity, we hypothesize that JAK2V617F-bearing MKs promote MPN stem cell function via altered TPO/MPL signaling.
Methods
JAK2V617F Flip-Flop (FF1) mice and Pf4-Cre mice were crossed to generate a MK cell lineage-specific human JAK2V617F knock-in mouse strain (Pf4/FF1). TPO or MPL was ablated in Pf4/FF1 by crossing Pf4/FF1 mice with TPO knock-out mice (TPOnull) or MPL knockout mice (MPLnull) to generate TPOnullPf4+FF1+ mice and MPLnullPf4+FF1+ mice. Animal experiments were performed in accordance with the Institutional Animal Care and Use Committee guideline.
Results
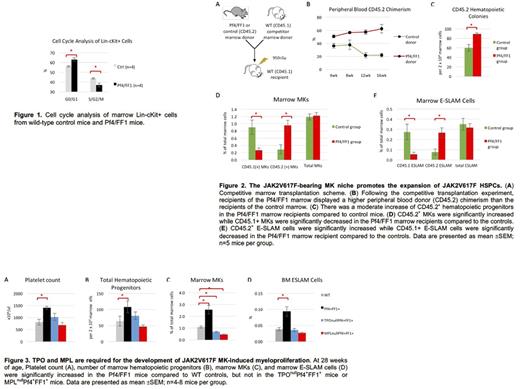
Previously, we have demonstrated that JAK2V617F-bearing MKs cause a murine myeloproliferative syndrome with HSPC expansion in a Pf4/FF1 murine model. Here, we studied the effects of the JAK2V617F-bearing MK niche on MPN stem cell function. First, we isolated LineagenegcKit+ (Lin-cKit+) HSPCs from 28wk old Pf4/FF1 mice and their age-matched littermate controls (Pf4-Cre) (n=4 in each group) and analyzed their cell cycle status using propidium iodide staining. We found that the percentage of Lin-cKit+ cells in the G0/G1 phase was moderately increased in the Pf4/FF1 mice compared to control mice (63% vs 56%, P=0.012), suggesting that HSPCs in the JAK2V617F-bearing MK niche are more quiescent than in the WT MK niche. (Figure 1)
Next, we performed a competitive repopulation assay in which both CD45.2 marrow cells from Pf4/FF1 mice or control mice were injected intravenously together with competitor CD45.1 WT marrow cells into lethally irradiated (950 cGy) CD45.1 recipients. During 16 weeks follow up post transplantation, recipients of Pf4/FF1 marrow cells displayed a higher peripheral blood donor (CD45.2) chimerism than the control mice. Marrow cell colony assays demonstrated a moderate increase of CD45.2+ hematopoietic progenitors in the Pf4/FF1 marrow recipients compared to control mice (1.5-fold, p = 0.0025). Marrow cell flow cytometry analysis revealed that, although there was no significant difference in the total CD41+ MK cell numbers between the two groups, CD45.2+ MKs were significantly increased (3.3-fold, P=0.012) while CD45.1+ MKs were significantly decreased (3.4-fold, P=0.024) in the Pf4/FF1 marrow recipient compared to the controls. Similarly, although the total numbers of marrow CD45+EPCR+CD48-CD150+ (E-SLAM) cells, which is a highly purified long-term repopulating HSPC population, were not changed between the two groups, CD45.2+ E-SLAM cells were significantly increased (3.5-fold, P=0.012) while CD45.1+ E-SLAM cells were significantly decreased (5.4-fold, P=0.024) in the Pf4/FF1 marrow recipient compared to the controls. Therefore, the JAK2V617F-bearing MK niche promoted the expansion of JAK2V617F HSPCs, probably at the expense of suppressing WT hematopoiesis. No significant difference in blood cell count, spleen weight, or total femoral cell count was observed between the Pf4/FF1 marrow recipients and control marrow recipients. (Figure 2)
To determine the role(s) of TPO/MPL signaling in the development of JAK2V617F MK-induced myeloproliferation, we ablated TPO or MPL in the Pf4/FF1 mice. At 28 weeks of age, in contrast to the Pf4/FF1 mice which developed thrombocytosis, increased numbers of hematopoietic progenitors, and a substantial expansion of MKs and HSPCs compared to age-matched WT controls, TPOnullPf4+FF1+ mice and MPLnullPf4+FF1+ mice did not display any of these features. (Figure 3) These data suggest that TPO and MPL are required for the development of JAK2V617F MK-induced myeloproliferation.
Conclusions
Our study establishes that JAK2V617F-bearing MKs form an important part of the MPN HSPC niche and TPO/MPL signaling is critical for the MK niche function.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal